Homocysteine, Vitamin B12, Folate and Cognitive Functions: A Systematic and Critical Review of the Literature (Medscape CME)
Authors and Disclosures
As an organization accredited by the ACCME, MedscapeCME requires everyone who is in a position to control the content of an education activity to disclose all relevant financial relationships with any commercial interest. The ACCME defines “relevant financial relationships” as financial relationships in any amount, occurring within the past 12 months, including financial relationships of a spouse or life partner, that could create a conflict of interest.
MedscapeCME encourages Authors to identify investigational products or off-label uses of products regulated by the US Food and Drug Administration, at first mention and where appropriate in the content.
Author(s)
Thomas Vogel, MD
Pôle de Gériatrie, Hôpitaux Universitaires de Strasbourg, Hôpital de la Robertsau, Pavillon Schutzenberger, Strasbourg Cedex, France
Disclosure: Thomas Vogel, MD, has disclosed no relevant financial relationships.
Nassim Dali-Youcef, PhD
Pôle de Biologie, Nouvel Hôpital Civil, Hôpitaux Universitaires de Strasbourg, Strasbourg Cedex, France
Disclosure: Nassim Dali-Youcef, PhD, has disclosed no relevant financial relationships.
Georges Kaltenbach, MD
Pôle de Gériatrie, Hôpitaux Universitaires de Strasbourg, Hôpital de la Robertsau, Pavillon Schutzenberger, Strasbourg Cedex, France
Disclosure: Georges Kaltenbach, MD, has disclosed no relevant financial relationships.
Emmanuel Andrès, MD
Service de Médecine Interne, Clinique Médicale B, Hôpital Civil, Hôpitaux Universitaires de Strasbourg, Strasbourg Cedex, France
Disclosure: Emmanuel Andrès, MD, has disclosed no relevant financial relationships.
CME Author(s)
Désirée Lie, MD, MSEd
Clinical Professor, Family Medicine, University of California, Orange; Director, Division of Faculty Development, UCI Medical Center, Orange, California
Disclosure: Désirée Lie, MD, MSEd, has disclosed no relevant financial relationships.
Editor(s)
Graham Jackson, MD, FESC, FRCP, FACC
Honorary Consultant Cardiologist, Cardiothoracic Centre, Guy’s and St. Thomas’ Hospital, London, United Kingdom
Disclosure: Graham Jackson, MD, FESC, FRCP, FACC, has disclosed that he has served as an academic advisor for Pfizer Inc.; Eli Lilly & Co., Inc.; Plethora Solutions Limited; Shire; and SERVIER, and has served as chairman of the Sexual Dysfunction Association. Dr. Jackson regularly gives sponsored lectures that are rigorously noncommercial.
Leslie Citrome, MD, MPH
Director, Clinical Research and Evaluation Facility, Nathan S. Kline Institute for Psychiatric Research, Orangeburg, New York; Professor of Psychiatry, New York University School of Medicine, New York, NY
Disclosure: Leslie Citrome, MD, MPH, has disclosed that he is a consultant for, has received honoraria from, or has conducted clinical research supported by Abbott Laboratories; AstraZeneca Pharmaceuticals LP; Avanir Pharmaceuticals; Azur Pharma Ltd.; Barr Pharmaceuticals, Inc.; Bristol-Myers Squibb Company; Eli Lilly & Co., Inc.; Forest Research Institute; GlaxoSmithKline; Janssen; Jazz Pharmaceuticals, Inc.; Pfizer Inc.; and Vanda Pharmaceuticals Inc.
Karen Costenbader, MD, MPH
Rheumatology and Immunology, Brigham and Women’s Hospital, Boston, Massachusetts
Disclosure: Karen Costenbader, MD, MPH, has disclosed no relevant financial relationships.
Serge Jabbour, MD
Associate Professor of Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolic Diseases, Department of Medicine, Jefferson Medical College/Thomas Jefferson University, Philadelphia, Pennsylvania
Disclosure: Serge Jabbour, MD, has disclosed that he has served on the speaker’s bureau for Amylin Pharmaceuticals, Inc. and Eli Lilly & Co., Inc.
Rubin Minhas
General Practitioner; Honorary Senior Lecturer, Faculty of Science, Technology, and Medical Studies, University of Kent, Kent, United Kingdom
Disclosure: Rubin Minhas, has disclosed no relevant financial relationships.
Matt Rosenberg, MD, BA
Medical Director, Mid-Michigan Health Centers, Jackson, Michigan
Disclosure: Matt Rosenberg, MD, BA, has disclosed that he has served as a consultant for Abbott Laboratories; Allergan, Inc.; Astellas Pharma, Inc.; GlaxoSmithKline; Novartis Pharmaceuticals Corporation; Ortho-McNeil-Janssen Pharmaceuticals, Inc.; Pfizer Inc.; Roche Laboratories Inc.; sanofi-aventis; and Verathon Inc. Dr. Rosenberg has also disclosed that he has received fees for non-CME services from Abbott Laboratories; Allergan, Inc.; Astellas Pharma, Inc.; GlaxoSmithKline; Esprit Pharma; Ortho-McNeil-Janssen Pharmaceuticals, Inc.; and Roche Laboratories Inc., and conducted research funded by sanofi-aventis.
Jagdish Sharma, FRCP, FESO
Sherwood Forest NHS Trust, Nottinghamshire, United Kingdom
Disclosure: Jagdish Sharma, FRCP, FESO, has disclosed no relevant financial relationships.
Anthony Wierzbicki, FACA, FACB
Senior Lecturer in Chemical Pathology, St. Thomas’ Hospital, London, United Kingdom
Disclosure: Anthony Wierzbicki, FACA, FACB, has disclosed that he has received support for travel from Abbott Laboratories; AstraZeneca Pharmaceuticals LP; Merck KGaA; and Merck Sharp & Dohme, and received lecture honoraria from Abbott Laboratories; AstraZeneca Pharmaceuticals LP; Bristol-Myers Squibb Company; Genzyme Corporation; Gilead Sciences, Inc.; Merck KGaA; Merck Sharp & Dohme; Pfizer Inc.; Roche Laboratories Inc.; Schering-Plough Corporation; and Solvay/Fournier. Dr. Wierzbicki is an advisory board member for Abbott Laboratories; AstraZeneca Pharmaceuticals LP; Bristol-Myers Squibb Company; Genzyme Corporation; Gilead Sciences, Inc.; Kowa Company Ltd.; LifeCycle Pharma; Merck KGaA; Merck Sharp & Dohme; Pfizer Inc.; Roche Laboratories Inc.; Schering-Plough Corporation; Solvay/Fournier; Surface Logix, Inc.; and Takeda Pharmaceuticals North America, Inc.
Mark Harries, MA, PhD, FRCP
Consultant in Medical Oncology, Guy’s and St. Thomas’ Hospital, London, United Kingdom
Disclosure: Mark Harries, MA, PhD, FRCP, has disclosed no relevant financial relationships.
Gabrielle Cremer, MD
Cremer Consulting, Strasbourg, France
Disclosure: Gabrielle Cremer, MD, has disclosed no relevant financial relationships.
Carol Peckham
Site Editorial Director, MedscapeCME
Disclosure: Carol Peckham has disclosed no relevant financial relationships.
Summary
Elevated serum homocysteine, decreased folate and low vitamin B12 serum levels are associated with poor cognitive function, cognitive decline and dementia. Despite evidence of an epidemiological association, randomised controlled trials did not provide any clear evidence so far that supplementation with vitamin B12 and/or folate improves dementia or slows cognitive decline, even though it might normalise homocysteine levels. In this report, we review the current knowledge on the relationship between homocysteine, folate and vitamin B12 levels and the way their disruption influences cognitive function in adults.
Introduction
The prevalence of cognitive impairment increases with advancing age, making it a major public health concern in ageing populations. Dementia represents the main cause of cognitive problems among the elderly people and is characterised by a progressive deterioration of cognitive skills, leading ultimately to difficulties performing daily activities. The number of people suffering from dementia might triple over the next 50 years, for reasons of an increase in the proportion of the oldest-old segment of the population[1]. Alzheimer disease (AD) accounts for about two-thirds of dementia. The prevalence of AD rises exponentially with age, affecting 50% of adults aged 95 years. Other causes of dementia are Lewy body dementia, vascular dementia, Parkinson’s disease-associated dementia, fronto-temporal dementia and reversible dementias. Measures for prevention of cognitive impairment are crucial for many reasons, most notably the lack of efficient therapies, the high prevalence of dementia and the devastating consequences of dementia for patients’ caregivers and healthcare system.
The most relevant risk factors for AD are advancing age, female gender and low level of education. Other non-genetic risk factors for AD include cardiovascular disease, stroke, hypertension, diabetes and traumatic head injury. Biomarkers associated with the risk of late-life cognitive impairment or dementia are not well-described and poorly understood. Despite this, it is interesting from a physiopathological perspective to take into account the nutritional component and diet.
An association between neuropsychiatric or neurological disorders (e.g. ‘subacute combined degeneration of the cord’) and vitamin B12 (cobalamin) deficiency has been recognised since pernicious anaemia was first described in 1849[2]. Inappropriate levels of vitamin B are a common cause of the reversible type of dementia. The association between vitamin B abnormalities and impaired cognitive function or dementia remains questionable as studies and interventional trials have reported conflicting results.
Several human studies have demonstrated a link between vitamin B deficiency (or hyperhomocysteinemia) and cognition. However, these studies were very heterogeneous in terms of the populations studied, cut-offs used to define vitamin B deficiency, definitions of vitamin B deficiency (serum levels, dietary intakes), assessments of cognitive impairment, definitions of cognitive impairment [incident dementia, prevalent dementia, type of dementia, mild cognitive impairment (MCI)] and study designs. Thus, comparing studies is difficult and caution is necessary when analysing and interpreting the results.
In this article, we distinguished three types of studies: cross-sectional studies, longitudinal studies, featuring a generally more adequate nutrient exposure and intervention studies with vitamin B supplementation.
General Information
Both vitamin B12 and folate (vitamin B9) are involved in a common metabolic pathway supplying essential methyl groups for DNA and protein synthesis (Figure 1). Vitamin B12 acts as a cofactor for methionine synthase that remethylates homocysteine to methionine by using 5-methyltetrahydrofolate as a methyl donor. Deficiency in either folate or vitamin B12 leads to an increase in total serum homocysteine concentrations. Vitamin B12 also acts as a cofactor for methylmalonyl-CoA mutase, which converts methylmalonyl-CoA to succinyl-CoA. Thus, vitamin B12 deficiency results in hypermethylmalonic acidemia. Hence, elevated methylmalonic acid (MMA) concentrations may indicate vitamin B12 deficiency, whereas hyperhomocysteinemia may reflect either vitamin B12 or folate deficiency.
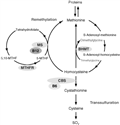 | Figure 1. Biological links between homocysteine, vitamin B12 and folate |
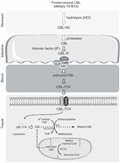
Vitamin B12 deficiency is frequent among the elderly people with a prevalence approaching 20%. Its metabolism is complex and includes multiple steps and processes (Figure 2). The two main causes of this deficiency are food cobalamin malabsorption and pernicious anaemia[3]. Folate deficiency is also often found among aged people and is primarily caused by dietary deficiency.
Figure 2.
Of particular concern, the diagnosis of vitamin B12 or folate deficiency remains difficult to establish as normal serum levels of vitamin B do not rule out a diagnosis of metabolic deficiency. An increased serum homocysteine concentration or high levels of MMA confirm a significant deficiency when vitamin B serum concentrations are borderline or equivocal.
Cross-sectional Surveys
More than 77 cross-sectional studies including more than 34,000 subjects[4] have reported a significant association between low blood levels of vitamin B9 and B12 (or high levels of homocysteine) and prevalent dementia[5–7]. This association was described for different types of cognitive impairment including vascular dementia, AD and the MCI. Some studies have evaluated the link between low vitamin B serum levels (or hyperhomocysteinemia) and specific cognitive domains, as shown by Feng et al.[8]. This study reported deficits in constructional ability using the Block Design Test, processing speed with the Symbol Digit Modality Test and memory using the Rey Auditory Verbal Learning Test. In a cross-sectional analysis of the Sacramento Area Latino Study on Aging, Miller et al. observed the same trend[9]. On the Contrary, several cross-sectional studies did not report a significant association between plasma levels of vitamin B or homocysteine and dementia[10,11].
All the studies reporting associations between cognitive function and homocysteine or vitamin B used a cross-sectional or case–control design. Hence, the authors were unable to exclude the possibility that such associations were rather a result than a cause of the disease. In our study of more than 400 patients (mean age 76 years) with B12 deficiency and hyperhomocysteinemia, the Folstein test, which estimates cognition, did not reveal any significant difference between the B12 deficiency group and an age-matched group [mean Folstein score (FS) = 16/30 for the deficient group vs. 17.5/30 for controls) (unpublished data from the CARE B12 team). Second, in more than 100 patients treated with oral cobalamin (cyanocobalamin between 0.125 and 1 mg per day) that normalised their vitamin B12 and homocysteine levels, the FS did not significantly differ from the pretreatment score (FS = 18.7/30 before vs. 16.2/30 after therapy).
Longitudinal Studies
More than 33 longitudinal studies (including more than 12,000 subjects) have analysed, with significant results, the association between low vitamin B12 and folate blood levels (or hyperhomocysteinemia) and incident dementia, incident cognitive impairment without dementia or cognitive decline[4,12]. However, not all studies confirmed the link between vitamin B, homocysteine and cognition, in terms of dementia, cognitive test scores or cognitive decline[13,14]. In addition, it is not clear whether the observed associations between homocysteine and cognition are causal or whether they are caused by homocysteine, independent actions of the vitamin B, or both. A 3-year longitudinal study (Veterans Affairs Normative Aging Study) documented an independent contribution of serum folate levels on cognitive scores (after adjustment for homocysteine and other vitamin ![]() and of dietary folate on cognitive functions (after adjustment for dietary vitamin
and of dietary folate on cognitive functions (after adjustment for dietary vitamin ![]() . These findings suggested that folate may have cognitive effects by mechanisms other than the elevation of homocysteine[15].
. These findings suggested that folate may have cognitive effects by mechanisms other than the elevation of homocysteine[15].
Concerning homocysteine, six prospective longitudinal studies reported that increased serum homocysteine was associated with an increased risk of dementia or cognitive decline, whereas four studies did not describe any association between homocysteine and cognition, which further fuels the debate.
There are concerns about the causal link between vitamin B deficiency or high homocysteine levels and cognitive functions. It has been suggested that the inability of demented patients to care for themselves may explain the vitamin B deficiency as a reflection of poor nutritional habits. In contrast, several studies employing other malnutrition indicators did not support this hypothesis[4,6].
Intervention Studies
Despite potential benefits of vitamin B supplementation for lowering homocysteine, the positive contribution of this supplementation to cognitive function among demented and non-demented patients remains debatable.
In the United States, the effect of folate supplementation was well illustrated in 1998, by the implementation of folate fortification. This was associated with a nearly 15% decline in serum homocysteine after 1998 (National Health and Nutrition Examination Survey in 1999–2002).
There was a large heterogeneity among vitamin B interventional studies with cognitive assessments in terms of dosage, routes of intervention (for vitamin B12), age and cognitive function
Folate Intervention
Only four randomised controlled trials, including patients with normal cognitive function, cognitive impairment and dementia, have evaluated the effect of folate supplementation on cognitive function. These trials included a total of 857 subjects with three different populations: demented subjects, cognitive impaired subjects and healthy subjects. Various doses of folate supplementation were used. Trial durations comprised between 5 weeks and 3 years and yielded discordant results.
Among cognitive impaired subjects with low folate serum levels, Fioravanti et al.[16] observed after 60 days of treatment a significant improvement of some scores of the Randt Memory Test in the folate-treated group compared with the placebo group. In the second trial that used a mixed factorial design in normal subjects, the authors observed in a post hoc analysis that folate-treated older women’s cognitive test scores improved (Rey Auditory-Verbal Learning Test)[17]. Controversially, a third small study including seven subjects with dementia reported no statistically significant differences between the supplemented group and the control group and noted a negative trend in specific test scores of the folate supplemented group[18]. Given the small number of subjects, study results need to be interpreted cautiously.
Finally, the 3-year randomised controlled FACIT trial included 818 older subjects (60 years old) with augmented plasma total homocysteine and normal serum vitamin B12 levels. The effect of folic acid supplementation on cognition was the secondary end-point. The 3-year change in memory, information processing speed and sensori-motor speed were significantly improved in the folic acid group in comparison to the placebo group[19].
In addition to these randomised controlled trials, a 17-week uncontrolled cohort study including demented subjects reported a significantly improved performance in different cognitive tests after folate supplementation[20].
For reasons of lacking evidence of folate action on cognition, any conclusion concerning effects is tentative, as they are dosage-dependent and may be related to specific cognitive functions.
Vitamin B12 Supplementation
Few studies have evaluated the cognitive benefits of vitamin B12 supplementation. Six randomised controlled trials assessed the effect of vitamin B12 intervention on cognitive function in humans. There is a large heterogeneity among trials regarding the cognitive status of participants, the doses and administration routes of vitamin B12, the duration of supplementation and the cognitive function assessment instruments used. Sample sizes ranged from 18 to 78 subjects receiving vitamin B12, and the duration of supplementation ranged from 4 weeks to 6 months. For most cognitive tests, there was no significant improvement in vitamin B12 supplemented patients as compared with the placebo group. Curiously, a statistically significant worsening of cognitive tests was reported in two studies. In 195 vitamin B12 deficient subjects of normal and impaired cognition, Eussen et al.[21] observed that improvement of the cognitive test score in the placebo group was significantly more marked than that of the vitamin B12 group (p = 0.0036). Similarly, another study reported a significant worsening of the ’12 words learning test’ score in a vitamin B12-treated population of 140 old patients with cognitive impairment and methylmalonic acidemia compared with the placebo group[22].
For reasons of heterogeneity of these controlled trials, no reasonable conclusion can be drawn regarding potential dose effects and differences in the effects on tests of different cognitive domains. In addition, several uncontrolled cohort studies assessed the effects of vitamin B12 intervention on cognitive function in humans with conflicting results.
Effects of Combination in Vitamin B Supplementation
Only seven trials reported data of combined vitamin intervention on cognition, in subjects with normal cognition, dementia or vascular disease (17–409 participants). Trial durations ranged from 12 weeks to 2 years. One study found a significant improvement in one of eight cognitive tests (Reitan trail-making test, part ![]() [23]. This does not sufficiently support a conclusion regarding any beneficial effects of the combined therapy. Four trials did not report any significant effect of vitamin B supplementation on cognition. In particular, a recent randomised controlled trial using data of the Alzheimer Disease Cooperative Study reported that a regimen of high-dose vitamin B vitamin supplements did not change the scores on the cognitive subscale of the Alzheimer Disease Assessment Scale in older subjects with mild to moderate AD and normal folate, vitamin B12 and homocysteine serum levels[24]. Of note, subjects in the placebo arm performed better in five of 25 tests used in two studies[22,25]. There was evidence across trials neither of potential dose effects nor of differences in the effect on tests of different cognitive domains.
[23]. This does not sufficiently support a conclusion regarding any beneficial effects of the combined therapy. Four trials did not report any significant effect of vitamin B supplementation on cognition. In particular, a recent randomised controlled trial using data of the Alzheimer Disease Cooperative Study reported that a regimen of high-dose vitamin B vitamin supplements did not change the scores on the cognitive subscale of the Alzheimer Disease Assessment Scale in older subjects with mild to moderate AD and normal folate, vitamin B12 and homocysteine serum levels[24]. Of note, subjects in the placebo arm performed better in five of 25 tests used in two studies[22,25]. There was evidence across trials neither of potential dose effects nor of differences in the effect on tests of different cognitive domains.
Discussion: Limitations
Many concerns and critical aspects must be taken into account to interpret results from the aforementioned studies. First, blood samples should be collected from fasting subjects. Indeed, fasting serum vitamin B9, vitamin B12 and homocysteine levels give a more accurate picture of the subject’s metabolic status. Moreover, the subject should have a light meal the evening prior to blood collection. Only few studies respected these recommendations. Second, there is no cut-off value for low serum cobalamin and low serum folate. Thus, some patients with ‘normal’ vitamin plasma levels may be misclassified in the vitamin-deprived group and statistical analysis may be incorrect. Third, vitamin B12 plasma levels are not sensitive enough to affirm or reject a deficiency. Other markers like MMA are more sensitive, but rarely used in studies. Furthermore, kidneys play an important role in homocysteine metabolism. The ageing process of kidneys and the decreased glomerular filtration rate, which are associated with a higher level of homocysteine serum concentration, should be taken into account. Despite this well-known association, there is no consensus on the upper normal limit for serum homocysteine levels in aged patients. Similarly, there is no consensus on the cut-offs of homocysteine or MMA to define vitamin B12 deficiency in an elderly population, in which impaired renal function can be an important confounding factor.
The potential confounding factors that may affect the serum levels of vitamin B12, folate and homocysteine, represent another limitation. Smoking, advanced age, male gender and alcohol intake or coffee consumption are factors that may increase serum homocysteine. It is also known that a low level of education and the apo-E ?4 allele are associated with AD. A frequent pitfall is to confound an acute confusion state with dementia, as subjects with AD frequently display delirium. The acute confusion may worsen the cognitive score. Other confounding factors are depression and, in longitudinal studies, baseline cognitive performance.
In addition, some biases exist, especially in longitudinal cohort studies, non-randomised trials or cross-sectional studies. In observational studies, another bias is the ‘healthy user’ bias, as the recorded exposure variable may be a marker of unrecorded data that influences the dependant variable. For example, subjects who take dietary supplements of vitamin B12 may display healthier behaviour that may be associated with cognitive performance. All these confounding factors have to be considered in the statistical analysis in studies.
Another limitation is the misclassification of the study subjects as cognitive impaired (or demented) or as healthy subjects. Subjects with normal scores on cognitive tests can have an AD at the preclinical state and they may develop an authentic AD, during the follow-up period, with multi-domain cognitive impairments. In contrast, subjects who perform poorly in the cognitive tests are not necessarily demented subjects, but may be depressed, taking sedative drugs, have sensorial impairment or have a low educational level.
Concluding Remarks
There is a substantial body of literature linking vitamin B and homocysteine with cognitive decline, dementia and mood (Figure 3). Despite this epidemiological association, there is to date no clear evidence from randomised controlled trials that supplementation with vitamin B12 and folate improves cognitive decline or dementia, even though it may normalise homocysteine levels[26]. This point of view is in accordance with the Cochrane review[27].
 | Figure 3 . Published studies linking vitamin B and homocysteine with cognitive decline, dementia and mood |
Figure 3 .
The results of the recent FACIT trial, which questioned the previous concept, may be an exception[20]. This large, randomised and controlled trial evaluated cognitive benefits of oral folate supplementation. The trial concluded that folate treatment significantly improved several cognitive domains that tend to decline with advancing age. For the first time, a long follow-up controlled study reported positive cognitive effects of folate treatment among healthy older subjects with enhanced serum homocysteine levels and normal vitamin B12 serum levels. Therefore, folate supplementation may be an interesting approach to prevent cognitive decline in elderly people. More definitive answers should come from large new trials that will test the clinical relevance of folate supplementation in older populations with either no cognitive impairment, MCI or dementia.